PHS Fellowship Supplemental V1.2
USER REQUIREMENTS Users are required to answer this form-specific Questionnaire and may need to input budget details to fulfill all the form requirements.
|
| ||||||
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
| ||
|
A-0 |
Application Type |
New, Resubmission, Renewal, Continuation, Revision |
|
| ||
|
B. Research training plan section | ||||||
|
| ||||||
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
| ||
|
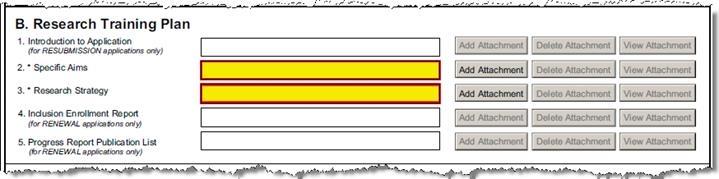
B-1 |
Intro to Application- (resubmissions only) |
Upload Proposal Attachment |
PHS_Fellow_IntroductionToApplication |
| ||
|
B-2 |
Specific Aims |
Upload Proposal Attachment -Required |
PHS_Fellow_SpecificAims |
| ||
|
B-3 |
Research Strategy |
Upload Proposal Attachment -Required |
PHS_Fellow_ResearchStrategy |
| ||
|
B-6 |
Inclusion Enrollment Report (renewals only) |
Upload Proposal Attachment |
PHS_Fellow_InclusionEnrollmentRpt |
| ||
|
B7 |
Progress Report Publication List (renewals only) |
Upload Proposal Attachment |
PHS_Fellow_ProgressReport_PubList |
| ||
|
|
B-8-1 Human Subjects Section | |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
| |||||||||||||||
|
|
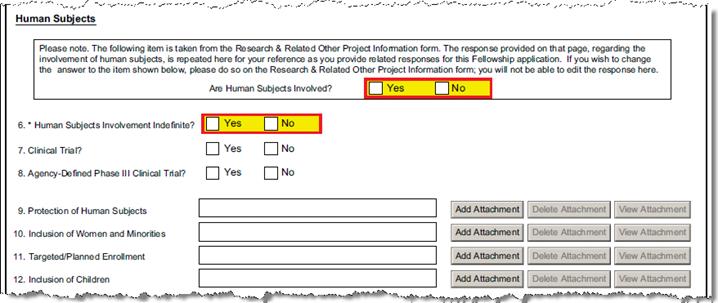
B-8.2 – 8.5 |
Are Human Subjects Involved? |
Special Review |
If Human Subjects special review is added, the form will check the Yes box. |
| |||||||||||||||
|
|
B-8.6 – 8.8 |
Human Subjects involvement indefinite? |
Questionnaire Q ID 1 |
A Yes or No answer is required. See Explanation, below. |
| |||||||||||||||
|
|
Explanation: If at the time of application, plans to involve human subjects are unknown, please check "Yes". In rare situations, applications are submitted with the knowledge that human subjects will be involved during the period of support, but plans are so indefinite that it is not possible to describe the involvement of human subjects in the application. The kinds of activities that lack definite plans are often institutional awards where the selection of specific projects is the institution's responsibility, research training grants, and projects in which the involvement of human subjects depends upon completion of instruments, animal studies, or purification of compounds. The Protection of Human Subjects upload is still required.
Policy: NIH Office of Extramural Research Human Subjects Website. This site provides, in one place, DHHS and NIH requirements and resources for the extramural community involved in human subjects research http://grants.nih.gov/grants/policy/hs/index.htm
|
| ||||||||||||||||||
|
|
B-9.1-9.3 |
Is the project a Clinical Trial? |
Questionnaire Q ID 2 |
Check the “Yes” or “No” to indicate whether the project is a clinical trial. See Explanation, below. |
| |||||||||||||||
|
|
Explanation: The NIH defines a clinical trial as a prospective biomedical or behavioral research study of human subjects that is designed to answer specific questions about biomedical or behavioral interventions (drugs, treatments, devices, or new ways of using known drugs, treatments, or devices). Clinical trials are used to determine whether new biomedical or behavioral interventions are safe, efficacious, and effective. Behavioral human subject research involving an intervention to modify behavior (diet, physical activity, cognitive therapy, etc.) fits this definition of a clinical trial. Human subject research to develop or evaluate clinical laboratory tests (e.g. imaging or molecular diagnostic tests) might be considered to be a clinical trial if the test will be used for medical decision making for the subject or the test itself imposes more than minimal risk for subjects.
Policy: Clinical Trials Registration in ClincialTrials.gov (Public Law 110-85): Competing Applications and Non-Competing Progress Reports NOTICE OD-08-023 http://grants.nih.gov/grants/guide/notice-files/NOT-OD-08-023.html
| |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
| |||||||||||||||
|
|
B-10.1-10.3 |
Is this an NIH-defined Phase III clinical trial? |
Questionnaire Q ID 3 |
Check the “Yes” or “No” to indicate whether the project is an NIH-defined Phase III clinical trial. See Explanation, below. |
| |||||||||||||||
|
|
Explanation: An NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of evaluating an experimental intervention in comparison with a standard or controlled intervention or comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included.
Policy: Clinical Trials Registration in ClincialTrials.gov (Public Law 110-85): Competing Applications and Non-Competing Progress Reports. NOTICE OD-08-023 http://grants.nih.gov/grants/guide/notice-files/NOT-OD-08-023.html
| |||||||||||||||||||
|
|
b-11 |
Protection of Human Subjects |
Upload Proposal Attachment |
PHS_Fellow_ProtectionOfHumanSubjects | ||||||||||||||||
|
|
b-12 |
Inclusion of Women and Minorities |
Upload Proposal Attachment |
PHS_Fellow_InclusionOfWomenAndMinorities | ||||||||||||||||
|
|
b-13 |
Targeted/Planned Enrollment |
Upload Proposal Attachment |
PHS_Fellow_TargetedPlannedEnrollment | ||||||||||||||||
|
|
B-14 |
Inclusion of Children |
Upload Proposal Attachment |
PHS_Fellow_InclusionOfChildren | ||||||||||||||||
|
|
Other Research Training Plan section | |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
Q |
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry | |||||||||||||||
|
|
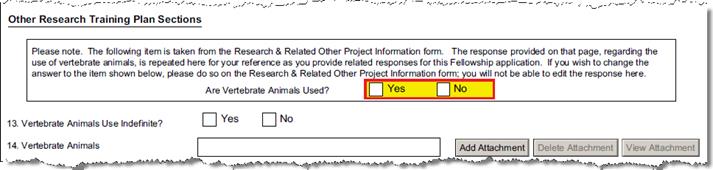
B-15.3-5 |
Are Vertebrate Animals Used? |
Special Review |
If an Animal Usage special review is added, the form will check the Yes box. | ||||||||||||||||
|
|
B-15.6-8 |
Will the inclusion of vertebrate animals use be indefinite? |
Questionnaire Q ID 4 |
A Yes or No answer is required. See Explanation, below. | ||||||||||||||||
|
|
Explanation: If animal involvement is anticipated within the period of award but plans are indefinite and it is not possible to describe the use of animals, check "Yes" and in the Research Training Plan: Vertebrate Animals narrative, provide an explanation and indicate when it is anticipated that animals will be used.
Policy: Refer to PHS Policy on Humane Care and Use of Laboratory Animals
| |||||||||||||||||||
|
|
B-16 |
Vertebrate Animals |
Upload Proposal Attachment |
PHS_Fellow_VertebrateAnimals | ||||||||||||||||
|
|
| |||||||||||||||||||
|
|
Q |
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry | |||||||||||||||
|
|
B-17 |
Select Agent Research |
Upload Proposal Attachment |
PHS_Fellow_SelectAgentResearch | ||||||||||||||||
|
|
B-18 |
Resource Sharing Plan |
Upload Proposal Attachment |
PHS_Fellow_ResourceSharingPlan | ||||||||||||||||
|
|
B-19 |
Respective Contributions |
Upload Proposal Attachment - Required |
PHS_Fellow_RespectiveContributions | ||||||||||||||||
|
|
B-20 |
Selection of Sponsor and Institution |
Upload Proposal Attachment - Required |
PHS_Fellow_SelectionSponsorInstitution | ||||||||||||||||
|
|
B-21 |
Responsible Conduct of Research |
Upload Proposal Attachment - Required |
PHS_Fellow_ResponsibleConductResearch | ||||||||||||||||
|
|
| |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
C. Additional Information | |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
| |||||||||||||||
|
|
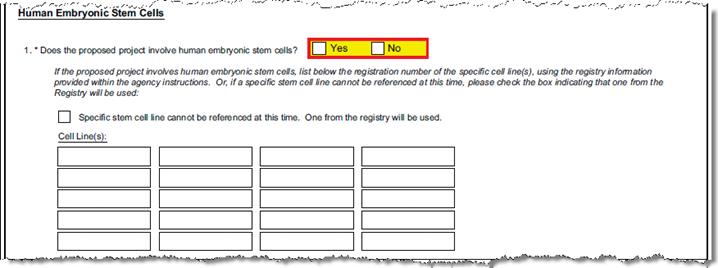
C-1.2-4 |
Does the proposed project involve human embryonic stem cells? |
Questionnaire Q ID 5 |
Indicate “Yes” if the proposed research involves human embryonic stem cells. | ||||||||||||||||
|
|
Explanation: See http://stemcells.nih.gov/index.asp for a definition of human embryonic stem cells.
Policy: See http://stemcells.nih.gov/policy/guidelines.asp for Federal policy on federally funded stem cell research.
| |||||||||||||||||||
|
|
C-1.6 |
Can a specific stem cell line be referenced at this time? |
Questionnaire Q ID 6 |
“N” answer affirms that an undefined registry cell line will be used. “Y” answer will require entering the cell IDs in the next question. | ||||||||||||||||
|
|
Explanation: See http://stemcells.nih.gov/research/registry/eligibilityCriteria.asp for additional information on stem cells.
Policy: See http://stemcells.nih.gov/policy/guidelines.asp for Federal policy on federally funded stem cell research.
| |||||||||||||||||||
|
|
C-1.5 |
List the registration number of the specific cell line(s) from the stem cell registry. |
Questionnaire Q ID 7 |
List the registration numbers of the cell lines in the spaces provided. The maximum allowed length of each registration number is four (4). | ||||||||||||||||
|
|
Explanation: List the registration numbers of the cell lines in the spaces provided. The maximum allowed length of each registration number is four (4).
Policy: See the stem cell registry found at: http://stemcells.nih.gov/registry/index.asp
| |||||||||||||||||||
|
|
| |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
| |||||||||||||||
|
|
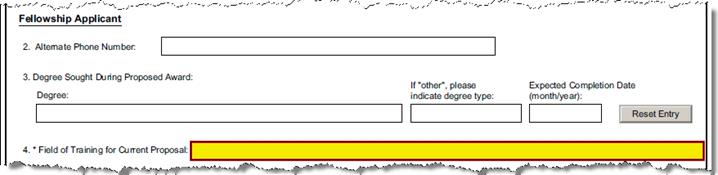
C-2.1 |
Candidates Alternate Phone Number |
Person Details |
Secondary Work Phone - Enter an alternate number (e.g., cell phone); field will be left blank if null. |
| |||||||||||||||
|
|
C-4.0 |
Degree Sought During Proposed Award: |
Questionnaire Q ID 42 |
Are you seeking a degree during the proposed award? “N” will proceed to Field of Training; “Y” requires degree date and type. |
| |||||||||||||||
|
|
C-4.3 |
Expected Completion Date |
Questionnaire Q ID 35 |
Enter the date the degree was earned in month/day/year format (MM/DD/YYYY). The specific date of the month is not important. The Month and Year data will be formatted to meet NIH requirements |
| |||||||||||||||
|
|
C-4.0 |
Degree Sought |
Questionnaire Q ID 99 |
If Yes, select the type of degree sought during the proposed award, from the list provided. If the degree being sought does not appear on the list, please select the most appropriate "other" degree type from the list. (List detailed in Appendix.) |
| |||||||||||||||
|
|
C-4.2 |
Other Degree Type |
Questionnaire Q ID’s 16, 17, 18, 19, 100, 21 |
If you have selected an "other" type as your degree sought, please provide the specific degree type here. |
| |||||||||||||||
|
|
C-5 |
Field of Training for Current Proposal: |
Questionnaire Q ID 22 |
Select subcategory field of training that best applies to the proposed award from the list; otherwise select “Subcategory Not Found”. |
| |||||||||||||||
|
|
|
Field of Training (broad) |
Questionnaire Q ID 23. |
Sponsor discourages use of the broader category descriptions, unless it’s truly the best fit; i.e. suitable subcategory cannot be found on list |
| |||||||||||||||
|
|
The form allows for up to four (4) current and prior support entries. The questionnaire asks the respondent if they had prior support; a “Yes” answer asks a series of questions to provide the form data. These series can repeat to supply the four detailed lines. A “No” response to current/prior support will present the next required question. | |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry | ||||||||||||||||
|
|
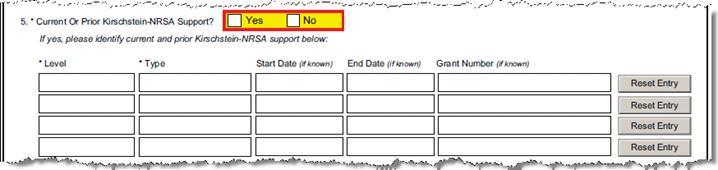
C-6.1 - 3 |
Current Or Prior Kirschstein-NRSA Support? |
Questionnaire Q ID 24 |
If yes, please identify current and prior Kirschstein-NRSA support in the following questions. Up to 4 awards can be identified. | ||||||||||||||||
|
|
C-6.4 |
Level |
Questionnaire Q ID 32 |
Select from list: Predoctoral or Postdoctoral | ||||||||||||||||
|
|
C-6.5 |
Type |
Questionnaire Q ID 33 |
Select from List: Individual or Institutional | ||||||||||||||||
|
|
C-6.6 |
Start Date |
Questionnaire Q ID 43 Q ID 44 |
If known, enter the start date of this support in the format MM/DD/YYYY. | ||||||||||||||||
|
|
C-6.7 |
End Date |
Questionnaire Q ID 49 Q ID 45 |
If known, enter the end date of this support in the format MM/DD/YYYY. | ||||||||||||||||
|
|
C-6.8 |
Grant Number |
Questionnaire Q ID 46 Q ID 27 |
If known, enter the grant number for this support. | ||||||||||||||||
|
|
Do you have another current or prior Kirschstein-NRSA support award to report? |
Questionnaire Q ID 31 |
Answer “Yes” to supply the required fields in the questions that follow. Answer “No” to proceed to the next required question. | |||||||||||||||||
|
|
| |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
| |||||||||||||||
|
|
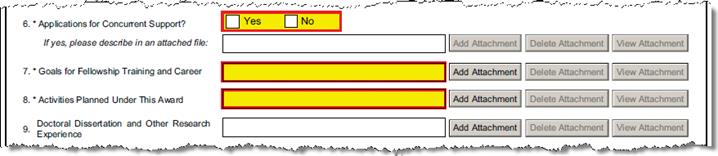
C-7.1 |
Applications for Concurrent Support? |
Defaults to No unless defined narrative is uploaded. |
Are there applications for other concurrent support for this candidate? If yes, upload the Concurrent Support narrative. | ||||||||||||||||
|
|
Explanation: If the candidate has applied or will be applying for other support that would run concurrently with the period covered by this application check “Yes” and include the type, dates, source(s) and amount in the uploaded attachment. The candidate must promptly report to the NIH IC to which this application is assigned any support resulting from other such applications. | |||||||||||||||||||
|
|
C-7.4 |
Concurrent Support |
Upload Proposal Attachment |
PHS_Fellow_ConcurrentSupport | ||||||||||||||||
|
|
C-8 |
Goals for Fellowship Training and Career |
Upload Proposal Attachment - Required |
PHS_Fellow_Goals_FellowshipTrainingCareer | ||||||||||||||||
|
|
C-9 |
Activities Planned Under This Award |
Upload Proposal Attachment - Required |
PHS_Fellow_ActivitiesPlanned | ||||||||||||||||
|
|
C-10 |
Doctoral Dissertation and Other Research Experience |
Upload Proposal Attachment |
PHS_Fellow_DocDissertOtherResExperience | ||||||||||||||||
|
|
Parameter 'PI_CITIZENSHIP_FROM_CUSTOM_DATA' supports this data element. Default value is 1. If the value is set to 1, uses field Citizenship Type in Person Extended Attributes table | |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry | ||||||||||||||||
|
|
C-11.1 |
Citizenship: |
Person Extended Attributes > Citizenship Type
|
Select from a look-up value provided: A: Non-U.S Citizen with Temporary Visa C: U.S. Citizen or noncitizen national N: Permanent Resident of the U.S. P: Permanent Resident of the U.S. - PENDING | ||||||||||||||||
|
|
| |||||||||||||||||||
|
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry | ||||||||||||||||
|
|
C-12.1 |
Change of Sponsoring Institution |
Questionnaire Q ID 28 |
Has this application been previously submitted by a different institution? Check YES, if this application reflects a change in grantee institution from that indicated on a previous application. | ||||||||||||||||
|
|
C-12.2 |
Name of Former Institution |
Questionnaire Q ID 29 |
Enter the name of the former institution. | ||||||||||||||||
|
|
Explanation: Per NIH, a former institution is not generally applicable to a "New" application. If you check YES, you will be prompted to provide the name in a follow-up question. | |||||||||||||||||||
|
|
Additional Narrative type for this form version 1-2. (prior instructions uploaded this file to the Other Project Info form.)
Consult the instructions provided in the Application Guide regarding the content of the Sponsor(s) and Co-Sponsor(s) section.
| |||||||||||||||||||
|
|
D-1 |
Sponsor(s) and Co-Sponsor(s) Information |
Upload Proposal Attachment - Required |
PHS_Fellow_Sponsor_CoSponsor_Info | ||||||||||||||||
|
| ||||||||||||||||||||
|
| ||||||||||||||||||||
|
E. BUDGET | ||||||||||||||||||||
|
| ||||||||||||||||||||
|
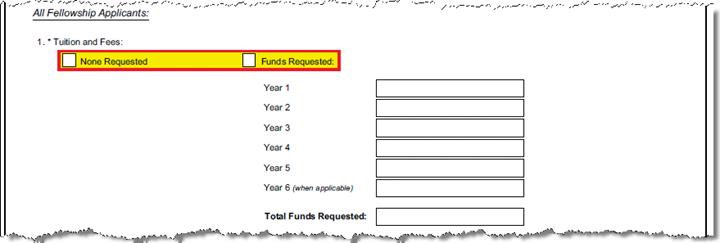
E-1 section |
If you are not requesting any tuition or fees in the detailed budget, "None Requested" will be selected. If you do have added Tuition in detailed budget "Funds Requested" will be selected and detail will map for each applicable year of support, in the fields provided. |
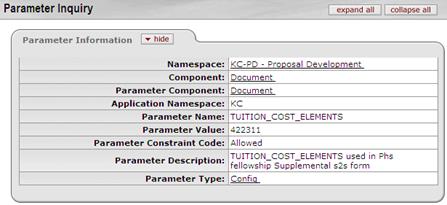
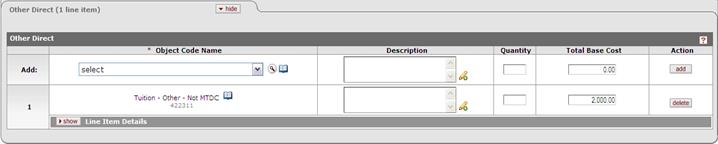
Budget:
Cost Element: 422311 Description: Tuition- Other– Not MTDC Line Item expense entered per budget period in the Cost Element defined for Tuition for this form. |
To populate this form the tuition must be budgeted using the parameterized cost element in Tuition_Cost _Elements. If Tuition is not budgeted, the “None Requested” box will be checked. Parameter: Tuition_Cost _Elements To maintain parameter: enter cost element in this method: (‘XXXXXX‘) e.g. parenthetical statement, single quote at start end of CE, Example: Warning: If Tuition is incorrectly budgeted (wrong cost element for this form), None Requested box will be checked. *current parameter default cost element is Tuition – Other – Not MTDC (422311) found in the Other Direct panel of Non-Personnel tab. | |||||||||||||||||
|
| ||||||||||||||||||||
|
Senior Fellowship Applicant section | |||
|
These questions will only appear upon “Yes” to the question “Is this a senior Fellowship Application. | |||
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
|
Senior Fellowship |
|
Questionnaire Q ID 36 |
Is this a Senior Fellowship Application? |
|
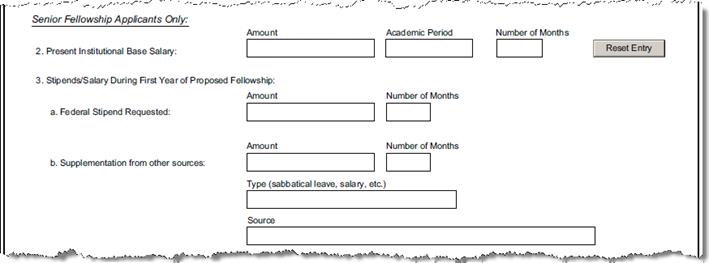
E-1-11 |
Present Institutional Base Salary Amount: |
Questionnaire Q ID 47 |
Please enter the dollar amount of your present institutional base salary |
|
E-1-13 |
Academic Period |
Questionnaire Q ID 48 |
Indicate the period of
time on which the salary is determined (e.g., academic year of 9 months,
full-time 12 months, etc. Select a value from the list
presented: |
|
E-1-14 |
Number of Months |
Questionnaire Q ID 50 |
Please enter the number of months you will receive the salary. Fractions of months (using two decimal places) may be expressed. |
|
E-2 |
Stipends/Salary During First Year of Proposed Fellowship |
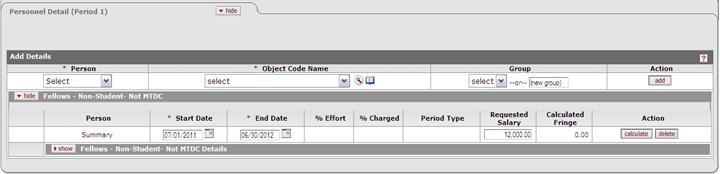
Budget: Period 1 Cost Element : 400315 Description: Fellows- Non-Student– Not MTDC |
To populate the Stipend Amount and Number of Months, this Fellow/Personnel expense must be in the Period 1 budget using the defined cost element. The amount and months are mapped from the Personnel Budget Details. If Stipend is not budgeted, the form fields will be zero. Parameter: Stipend_Cost _Elements Warning: If Stipend is incorrectly budgeted (wrong cost element or no person details), the values will not publish. * current parameter default cost element is Fellows-Non-Student-Not MTDC (400315) found in the Personnel tab. |
|
| |||
|
E-2 a1 |
Supplemental Amount |
Questionnaire Q ID 38 |
Are you receiving any supplementation from other sources? (Numeric value, no commas or non-numeric characters). |
|
E-2 a2 |
Supplemental Number of Months |
Questionnaire Q ID 39 |
Enter the number of months receiving the supplemental funds. (Numeric value, no commas/non-numeric characters). |
|
E-2b-1 |
Supplemental Type |
Questionnaire Q40 |
What is the type of the supplemental funding? |
|
E-2b-2 |
Sources: |
Questionnaire Q41 |
What is the source of the supplemental funding?? |
|
| |||
|
Section |
Field Name |
Options/Answers |
Upload Narratives or Data entry |
|
E-0 |
Appendix |
Upload Narrative Attachment |
PHS_Fellow_Appendix This narrative type requires a Description/Title. |
Form End Notes:
Form V 1-2
New Questionnaire (ID 4) created to support the 1-2 form version; a new value list (Graduate Level Degree 1-2 ) is provided for the selection of Graduate Level Degree 1-2.
New narrative type added: PHS_Fellow_Sponsor_CoSponsor_Info
New Argument Value list for Graduate Level Degree
Updated the Senior Fellow Federal Stipend Number of Months mapping from Personnel Budget Details data.
Prior Form Versions:
Form V 1-1: Only difference for this form version is there is no narrative type of PHS_Fellow_Sponsor_CoSponsor_Info, which was added for V1.2, all other field mapping and attachments are the same.
Appendix:
1. Degrees: maintained in Value List. Graduate Level Degrees 1-2
Masters
MA: Master of Arts
MBA: Master of Business Administration
MLS: Master of Library Science
MPA: Master of Public Administration
MPH: Master of Public Health
MS: Master of Science
MSN: Master of Science In Nursing
Academic Doctorate
DC: Doctor of Chiropractic
DNSC: Doctor of Nursing Science
DPH: Doctor of Public Health
DRPH: Doctor of Public Health
DSC: Doctor of Science
EDD: Doctor of Education
EGND: Foreign Doctor Engineering
JD: Doctor of Juris Prudence
PHD: Doctor of Philosophy
SCD: Doctor of Science
Professional Doctorate
DDS: Doctor of Dental Surgery
DMD: Doctor of Medical Dentistry
DO: Doctor of Osteopathy
DPM: Doctor of Podiatric Medicine
DSW: Doctor of Social Work
DVM: Doctor of Veterinary Medicine
MB: Foreign - Bachelor of Medicine
MBBS: Foreign - Bachelor of Medicine and Surgery
MD: Doctor of Medicine
ND: Doctor of Naturopathy
OD: Doctor of Optometry
PHMD: Doctor of Pharmacy
PSYD: Doctor of Psychology
VMD: Doctor of Veterinary Medicine
Other Degree(s)
MOTH: Other Masters Degree
DOTH: Other Doctorate
DDOT: Other Doctor of Medical Dentistry
MDOT: Other Doctor of Medicine
VDOT: Other Doctor of Veterinary Medicine
OTH: Other
2. Field of Training C-4. FOT Values: Excel table in DAT.
|
Predominantly Non-Clinical or Lab-Based Research Training |
|
1100 BIOCHEMISTRY |
|
1110 Biological Chemistry |
|
1120 Bioenergetics |
|
1130 Enzymology |
|
1140 Metabolism |
|
1200 BIOENGINEERING |
|
1210 Bioelectric/Biomagnetic |
|
1220 Biomaterials |
|
1230 Biomechanical Engineering |
|
1240 Imaging |
|
1250 Instrumentation and Devices |
|
1260 Mathematical Modeling |
|
1270 Medical Implant Science |
|
1280 Nanotechnology |
|
1290 Rehabilitation Engineering |
|
1310 Tissue Engineering |
|
1400 BIOPHYSICS |
|
1410 Kinetics |
|
1420 Spectroscopy |
|
1430 Structural Biology |
|
1440 Theoretical Biophysics |
|
1500 BIOTECHNOLOGY |
|
1510 Applied Molecular Biology |
|
1520 Bioprocessing and Fermentation |
|
1530 Metabolic Engineering |
|
1600 CELL AND DEVELOPMENTAL BIOLOGY |
|
1610 Cell Biology |
|
1620 Developmental Biology |
|
1700 CHEMISTRY |
|
1710 Analytical Chemistry |
|
1720 Bioinorganic Chemistry |
|
1730 Bioorganic Chemistry |
|
1740 Biophysical Chemistry |
|
1750 Medicinal Chemistry |
|
1760 Physical Chemistry |
|
1770 Synthetic Chemistry |
|
1900 ENVIRONMENTAL SCIENCES |
|
2000 GENETICS |
|
2010 Behavioral Genetics |
|
2020 Developmental Genetics |
|
2030 Genetic Epidemiology |
|
2040 Genetics of Aging |
|
2050 Genomics |
|
2060 Human Genetics |
|
2070 Molecular Genetics |
|
2080 Population Genetics |
|
2200 IMMUNOLOGY |
|
2210 Asthma and Allergic Mechanisms |
|
2220 Autoimmunity |
|
2230 Immunodeficiency |
|
2240 Immunogenetics |
|
2250 Immunopathology |
|
2260 Immunoregulation |
|
2270 Inflammation |
|
2280 Structural Immunology |
|
2290 Transplantation Biology |
|
2310 Vaccine Development |
|
2400 MICROBIOLOGY AND INFECTIOUS DISEASES |
|
2410 Bacteriology |
|
2420 Etiology |
|
2430 HIV/AIDS |
|
2440 Mycology |
|
2450 Parasitology |
|
2460 Pathogenesis of Infectious Diseases |
|
2470 Virology |
|
2600 MOLECULAR BIOLOGY |
|
2800 NEUROSCIENCE |
|
2810 Behavioral Neuroscience |
|
2820 Cellular neuroscience |
|
2830 Cognitive neuroscience |
|
2840 Communication Neuroscience |
|
2850 Computational Neuroscience |
|
2860 Developmental Neuroscience |
|
2870 Molecular Neuroscience |
|
2880 Neurochemistry |
|
2890 Neurodegeneration |
|
2910 Neuropharmacology |
|
2920 Systems/Integrative Neuroscience |
|
3100 NUTRITIONAL SCIENCES |
|
3200 PHARMACOLOGY |
|
3210 Molecular Pharmacology |
|
3220 Pharmacodynamics |
|
3230 Pharmacogenetics |
|
3240 Toxicology |
|
3300 PHYSIOLOGY |
|
3310 Aging |
|
3320 Anesthesiology (basic science) |
|
3330 Endocrinology (basic science) |
|
3340 Exercise Physiology (basic science) |
|
3350 Integrative Biology |
|
3360 Molecular Medicine |
|
3370 Physiological Optics |
|
3380 Reproductive Physiology |
|
3500 PLANT BIOLOGY |
|
3600 PSYCHOLOGY, NON-CLINICAL |
|
3610 Behavioral Communication Sciences |
|
3620 Behavioral Medicine (non-clinical) |
|
3630 Cognitive Psychology |
|
3640 Developmental and Child Psychology |
|
3650 Experimental & General Psychology |
|
3660 Mind-Body Studies |
|
3680 Neuropsychology |
|
3690 Personality and Emotion |
|
3710 Physiological Psychology & Psychobiology |
|
3720 Psychology of Aging |
|
3730 Psychometrics |
|
3740 Psychophysics |
|
3750 Social Psychology |
|
3900 PUBLIC HEALTH |
|
3910 Disease Prevention and Control |
|
3920 Epidemiology |
|
3930 Health Economics |
|
3940 Health Education |
|
3950 Health Policy Research |
|
3960 Health Services Research |
|
3970 Occupational and Environmental Health |
|
4100 RADIATION, NON-CLINICAL |
|
4110 Nuclear Chemistry |
|
4120 Radiation Physics |
|
4130 Radiobiology |
|
4200 SOCIAL SCIENCES |
|
4210 Anthropology |
|
4220 Bioethics |
|
4230 Demography & Population Studies |
|
4240 Economics |
|
4250 Education |
|
4260 Language and Linguistics |
|
4270 Sociology |
|
4400 STATISTICS AND/OR RESEARCH METHODS AND/OR INFORMATICS |
|
4410 Biostatistics and/or Biometry |
|
4420 Bioinformatics |
|
4430 Computational Science |
|
4440 Information Science |
|
4450 Clinical Trials Methodology |
|
4600 TRAUMA, NON CLINICAL |
|
5000 OTHER, Predominantly Non-Clinical or Lab-Based Research Training |
|
Predominantly Clinical Research Training (can include any degree) |
|
6100 ALLIED HEALTH |
|
6110 Audiology |
|
6120 Community Psychology |
|
6130 Exercise Physiology (clinical) |
|
6140 Medical Genetics |
|
6150 Occupational Health |
|
6160 Palliative Care |
|
6170 Physical Therapy |
|
6180 Pharmacy |
|
6190 Social Work |
|
6210 Speech-language Pathology |
|
6211 Rehabilitation |
|
6400 DENTISTRY |
|
6500 CLINICAL DISCIPLINES |
|
6510 Allergy |
|
6520 Anesthesiology |
|
6530 Behavioral Medicine (clinical) |
|
6540 Cardiovascular Diseases |
|
6550 Clinical Laboratory Medicine |
|
6560 Clinical Nutrition |
|
6570 Clinical Pharmacology |
|
6580 Complementary and Alternative Medicine |
|
6590 Clinical Psychology |
|
6610 Connective Tissue Diseases |
|
6620 Dermatology |
|
6630 Diabetes |
|
6640 Gastroenterology |
|
6650 Endocrinology |
|
6660 Immunology |
|
6670 Gene Therapy (clinical) |
|
6680 Geriatrics |
|
6690 Hematology |
|
6710 HIV/AIDS |
|
6820 Infectious Diseases |
|
6830 Liver Diseases |
|
6840 Metabolic Diseases |
|
6850 Nephrology |
|
6860 Neurology |
|
6870 Ophthalmology |
|
6880 Nuclear Medicine |
|
6890 OB-GYN |
|
6910 Oncology |
|
6920 Orthopedics |
|
6930 Otorhinolarynology |
|
6940 Preventive Medicine |
|
6950 Radiation, Interventional |
|
6960 Pulmonary Diseases |
|
6970 Radiology, Diagnostic |
|
6980 Rehabilitation Medicine |
|
6990 Psychiatry |
|
7110 Surgery |
|
7120 Trauma |
|
7130 Urology |
|
7300 PEDIATRIC DISCIPLINES |
|
7310 Pediatric Endocrinology |
|
7320 Pediatric Hematology |
|
7330 Pediatric Oncology |
|
7340 Pediatric, Prematurity & Newborn |
|
7500 NURSING |
|
7700 VETERINARY MEDICINE |
|
8000 OTHER, Predominantly Clinical Research Training |