Questionnaire
The Questionnaire page of the Protocol document displays online forms that allow you to record compliance information that must be collected at state, local and institutional levels and from sponsor to sponsor.
Association With Other Documents/Modules: The functionality used to create and maintain questionnaires in KC is shared by multiple modules, but the data is not. While a questionnaire can be associated with different KC modules, including Proposal, Conflict of Interest, Institutional Animal Care and Use, Institutional Review Board, and Award, the data collected in one module is entirely independent from data collected in another module.
Questionnaire Creation and Maintenance: Because questions are derived from government and institutional policies that are often subject to change, Administrators have the ability to maintain the question content and related usage information in the Questionnaire, Question, Question Category, and Question Type maintenance documents, which are accessible from the Shared group on the Maintenance menu.
Overview
The Questionnaire page displays categorized, expandable sections that display questions and standard selection/entry tools to answer them, with a save button at the bottom of the page that enables you to save your answer selections and entries.
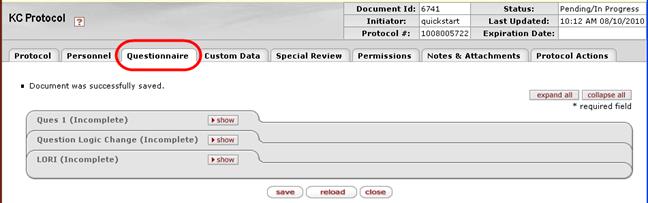
Figure 1119 Protocol Document – Questionnaire Page Layout
Table 333 Protocol Document – Questionnaire Page Component Descriptions
|
Page Component |
Brief Description |
|
Expand/Collapse All Buttons |
Control of the display view of tabbed sections of the page |
|
Categorized Sections |
Display of questions, show/hide control, entry/selection tools for answering the questions, print ability |
|
Action Buttons |
Command the system to, for example, save your answers to the document |
Question Sections
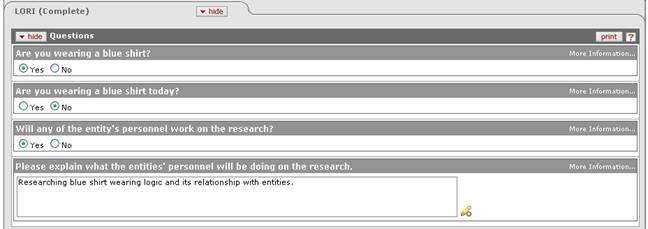
Figure 1120 Protocol Document, Questionnaire Page - Questions Section Example
Answering A Questionnaire
 To
answer the questions:
To
answer the questions:
1. Make selections and type to enter text as you would a typical online survey.
2. Click the save  button when finished.
button when finished.
Standard selection and entry tools include radio buttons and checkboxes for selection of options for Yes/No, True/False, and Multiple Choice questions; and textbox fields for input of information to answer Text Entry questions.
|
|
For more information about using the standard online form features to answer questions, see “Selection, Entry & Action Tools” on page Error! Bookmark not defined. in User Interface Orientation. |
|
|
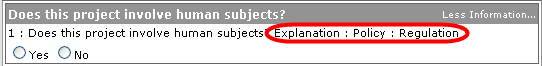
Branching Concept: Answers to particular questions can cause additional questions to appear. Such logic is driven by business rules embedded into the system so that an answer given to a previous question will dynamically add one or more new questions (or new tabbed sections containing multiple questions) to the Questionnaire. More Information To obtain more information about a question, click on the ‘More Information’ text to the right of the question, which causes additional information about the question to appear in the answer area.
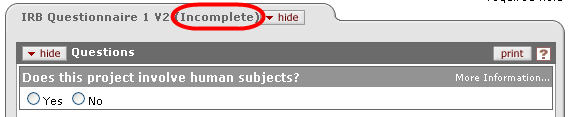
Partial Completion An Incomplete notification is displayed in the tabbed section heading to the right of the section label.
|
|
|
If you have not completed a Questionnaire, you are able to save your work and return to complete it at a later time prior to submission. Additionally, you have the ability to modify previous responses as desired. |
Print Capability: Click the print  button in the upper, right of
the section label if desired.
button in the upper, right of
the section label if desired.
Example Scenarios
COI Disclosure: One example of a regulatory Questionnaire is the conflict of interest (COI) financial disclosure form that has to be filed by Principal Investigators (PIs) and by some higher level academic administrators such as Deans and Vice Presidents. The COI disclosure is often presented as an electronic or paper-based Questionnaire. It is unique to individual institutions operating with their own state laws and will have numerous questions that are confidential in nature related to the financial interests of the PI or high-level administrator. The questions are of various answer types including yes/no, free text, and multiple choice. Many questions only have to be answered if applicable to the person or situation, otherwise they can be skipped. Significant branching is required as more detailed information is required, based upon the answer to a specific question. A COI Questionnaire, once defined and approved at an institution, is distributed and completed by all who are required to do so. This is usually done on an annual basis and is triggered by a specified date. It can also be activity driven by the creation of a new research proposal or protocol. Many organizations use both annual and activity based (event driven) COI disclosures and the questions on each are different. The questions and answers from the COI disclosure need to be stored and accessible to those determined to have permissions to view them with an audit trail for historical purposes. Because of the confidential nature, the stored data should be highly secured with limited access.
IRB/IACUC Application: Other examples are the Institutional Review Board (IRB) and Institutional Animal Care and Use (IACUC) initial review applications to use humans and animals in research and teaching activities. The IRB/IACUC application is filed by principal investigators (PIs) and secondary investigators who are conducting research activities with human/animal participants. The IRB/IACUC application is often presented as an electronic or paper-based Questionnaire. The application is a mechanism to gather information about a research process that is being proposed by a PI, so that a board of experts can make a decision about the participant's safety. It will have numerous questions exploring the proposed research, methods, and participants. The questions are of various answer types including yes/no, free text, date, checklist, and multiple choice. Many questions only have to be answered if applicable to the type of research or situation, otherwise they can be skipped or branching to another part of the application will occur. An IRB/IACUC application will be required for all proposals and protocols that involve the use of human/animal participants. The questions and answers from the COI (IRB/IACUC) disclosure need to be stored and accessible to IRB/IACUC administrators and those determined to have (administrative rights) permissions to view and update them. Security needs to ensure certain individuals only have access to protocols they are involved while others would need to have access on a need to know basis.
Conditional Branching Example: An example of conditional branching in an IRB Questionnaire might be to have a question such as "Do you provide clinical care to patients?" If answered yes, specific clinical care conflict of interest questions will be presented. Another example might be from the IACUC - "Are you using X species of animals?" If yes, questions specific to the X species might be presented. While very similar to the IRB Questionnaires, the IACUC needs to have significant branching--the euthanasia agent for a specific species, anesthesia for each species, room locations, restraining devices, etc.