Close Enrollment
The Close Enrollment action allows an IRB administrator to indicate that no new people will be enrolled for the human subjects research as indicated in the IRB protocol. The protocol remains active. The IRB administrator action is typically preceded by a researcher request to close enrollment for the protocol.
Table 353 Protocol Document, Protocol Actions Page, Request an Action Section, Close Enrollment Action – Action Attributes
|
Action attributes |
Description |
|
Who can perform action |
IRB Administrators are allowed to perform this action. |
|
Protocol state prior to action |
Prior to the action being performed, the protocol must be in the following state:
The protocol status must be Active - Open to Enrollment The submission status can be in any state.
|
|
Protocol state after action |
After the action is performed
The protocol status changes to Active - Closed to Enrollment The submission status changes to Closed for Enrollment |
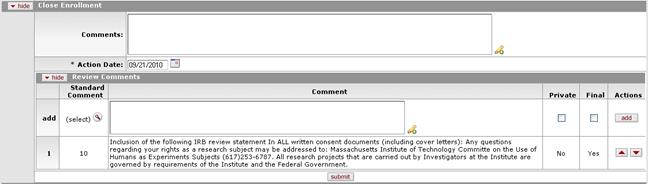
Figure 1156 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Close Enrollment Layout
Table 354 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Close Enrollment Field Descriptions
|
Field |
Description |
|
Close Enrollment | |
|
Comments |
Enter textual comments pertaining to the rationale
behind the close enrollment action. Click within the text box
(or press the tab |
|
Action Date |
Select the date you want the close enrollment action to
become effective. Click the calendar |
|
Review Comments |
|
 key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information. Click the add note
key from a previous field) to
relocate the cursor to the field, and then type (or paste from virtual
clipboard) to enter text in the box as necessary to provide the
appropriate information. Click the add note  icon to view/edit/paste text
in a new browser window, then click the continue button to return to the
text entry field in the document. After saved, click the green arrow
icon to view/edit/paste text
in a new browser window, then click the continue button to return to the
text entry field in the document. After saved, click the green arrow
 symbol to view full
text in a separate browser window.
symbol to view full
text in a separate browser window.
