Request an Action
The Request an Action section of the Protocol Actions page displays subsections for each available action. Available actions differ depending on factors such as previously-performed actions, pending action requests, the role of the logged-in user, and document status.
The standard initial actions available when a Researcher is working with a new Protocol document (with a status of Pending/In Progress) are:
• Submit for Review
• Delete Protocol, Amendment, or Renewal
Information about how to use these subsections follow.
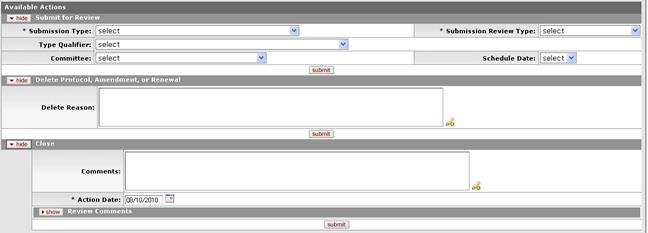
Figure 1140 Protocol Document, Protocol Actions Page, Request An Action Section, Initial Available Actions Sections Layout
Table 339 Protocol Document, Protocol Actions Page, Request An Action Section - Initial Available Actions Field Descriptions
|
Field |
Description |
|
Submit for Review | |
|
Submission Type |
Required. Select one of the following options as
appropriate: Initial
Protocol Application for Approval, Continuing Review/Continuation without
Amendment, Amendment, Response to Previous IRB Notification,
or Continuing
Review/Continuation with Amendment. Use the drop-down |
|
Type Qualifier |
Select one of the following options as
appropriate: Request for
Eligibility Exception, Training Certification, Unanticipated Problems,
DSMB Report, Annual Report, Annual Scheduled by IRB,
Contingent/Conditional Approval/Deferred Approval/Non-Approval,
Eligibility Exceptions/Protocol Deviations, AE/UADE, Compliant, Deviation,
Protocol-related COI Report, or Self report for
Noncompliance. Use the drop-down |
|
Committee |
Select the desired option from the list of committee
names. Use the drop-down |
|
Submission Review Type |
Select one of the following: Full, Expedited, Exempt, Limited/Single
Use, IRB Review not required, Response, or FYI as appropriate. Use
the drop-down |
|
Schedule Date |
Select the desired date from the list that is
automatically populated with available meeting dates based on your
selection in the Committee field. Use the drop-down |
|
|
Click this button to command the system to submit the review request you selected and notify recipients of the action request, which will appear in their respective action lists. |
|
Delete Protocol, Amendment, or Renewal | |
|
Delete Reason |
Click within the text box (or press the tab |
|
|
Click this button to record the reason you entered and
initiate the deletion action, then click the yes |
|
|
Configure Committee & Schedule display: The system allows implementing institutions to configure the display of committee and schedule during protocol submission. The system parameter IRB_COMM_SELECTION_DURING_SUBMISSION allows for the following configurations. M – Mandatory selection of committee and schedule during protocol submission O – Optional selection of committee and schedule during protocol submission. D – Do not display or allow selection of committee and schedule during protocol submission. |
|
|
Configure Submission Review Type: The system allows implementing institutions to control whether the values appearing in Submission Review Type should be available to only the IRB administrator or both the researcher and the IRB administrator. Within the maintenance table Protocol Review Type, a Global Flag value of “Y” indicates the submission review type value will be visible to both the IRB administrator and the researcher. A Global Flag value of “N” indicates that the submission review type value will be visible only to the researcher. |
Subsequent Available Actions
The list of Available Actions changes dynamically based on the role of the user and the current state of the Protocol document. For example, the Researcher will see a different set of actions than an IRB Administrator; and before the Protocol document is approved, there will be a different set of actions than after it is approved.
|
|
“Follow-up actions” appear in the Request an Action section after certain actions are performed. Only those actions which can logically be performed given the new state of the Protocol document dynamically appear in the refreshed list of available actions. The logged-in user’s role and associated permissions also dictate the actions that are available. |
Depending on your role, previously-requested actions and the current document status, additional actions that can be requested appear as subsections with show/hide buttons to control their display, as shown in the following example:
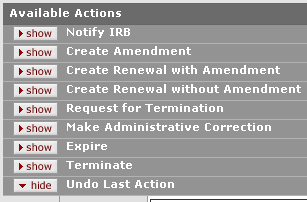
Figure 1141 Protocol Document, Protocol Actions Page, Request an Action Section, Subsequent Available Actions Example
How-To Quick Reference: The following table identifies various Available Actions that are possible, and briefly summarizes and describes how to execute the action using the entry, selection and action tools provided in each corresponding subsection.
|
|
For more detailed information by role and status, including descriptions of each field, see “Available Actions” on page Error! Bookmark not defined. which follows the table. |
Table 340 Protocol Document, Protocol Actions Page, Request an Action Section, Subsequent Available Actions Descriptions (Alphabetical)
|
Available Actions Subsection |
Summarized Usage Description |
|
Approve |
After recording a vote on a motion to approve, the IRB Administrator records the decision to grant approval, enters the approval date and comments. Expiration date will be generated based on approval date, but can be modified. Risk level can be recorded. |
|
Assign Reviewers |
Click the desired checkbox for each reviewer you want to select, choose either primary or secondary for each, then click the submit button. |
|
Assign to Agenda |
Click the Assign this protocol to an agenda checkbox, type to enter comments, select an Action Date as desired; optionally add Review comments, then click the submit button. |
|
Assign to Committee and Schedule |
Select a Committee and Schedule Date from the dropdown lists, then click the submit button. |
|
Close |
Enter comments, review comments and an effective date. |
|
Close Enrollment |
This is the IRB Administrator’s reaction to the Researcher’s request to close enrollment of study participants by the PI and it changes the status of the document to Active – Closed for Enrollment. This action can also be taken by the IRB Administrator without a request from the Researcher. |
|
Create Amendment |
Type to enter text in Amendment Summary; select appropriate checkbox(es) for the portion of the Protocol document in need of amendment in Amend; then click the create button. A new Protocol document is created and you are taken to the selected portion. An amendment is a request by the researcher to make a change to a previously approved protocol. This change may be procedural or informational. Probably the most common amendment is a change to the personnel working on the project. For an amendment KC will allow changes to the data elements in the protocol in specific sections identified by the researcher. It is possible to have more than one amendment outstanding at the same time, but to avoid data collisions KC does not allow two amendments of the same section of the protocol to be outstanding at the same time. |
|
Create Renewal with Amendment |
Type to enter text in Amendment Summary; select appropriate checkbox(es) for the portion of the Protocol document in need of amendment in Amend; then click the create button. A new Protocol document is created and you are taken to the selected portion. Changes to the data elements and an extension of the expiration date will result upon approval. |
|
Create Renewal without Amendment |
Click the create button. A renewal (also referred to as a continuation) is a request (usually annually) to continue work on a previously approved project. A renewal would not include any change in process or information. It may require some sort of progress report or statement. The acceptance of the renewal by the IRB committee will generally extend the expiration date (default in KC is to extend by 1 year). For a renewal without amendment KC will not allow any data elements in the protocol to be modified. |
|
Data Analysis Only |
Response to Researcher request for Data Analysis Only, this action indicates that that the human participant portion of the study is complete and only data analysis remains to be completed. This action can also be taken by the IRB Administrator without a request from the Researcher. |
|
Defer Action |
Enter comments pertaining to the reason for deferment, specify an Action Date for the deferment action to become effective, optionally add Review Comments, then click the submit button. |
|
Delete Protocol, Amendment, or Renewal |
When the Protocol document has never been submitted, this allows you to delete it from the system. |
|
Expedited Approval |
IRB Administrator records decision to grant expedited approval. Enters the approval date and comments. Expiration date will be generated based on approval date, but can be modified. Risk level can be recorded. |
|
Expire |
Enter comments that summarize the reason for the expiration, then select an action date for the expiration to become effective. |
|
Grant Exemption |
Optionally enter review comments and action comments, modify action date, and add to a meeting agenda to grant an exemption to a previously-submitted Protocol document. |
|
Make Administrative Correction |
Type to enter comment text explaining the purpose for the correction, then click the edit button. Make corrections in the appropriate sections, then select Apply Administrative Correction checkbox and click the submit button. |
|
Manage Review Comments |
Enter review comments without performing any additional action on the protocol. |
|
Modify Submission Request |
Select different options for Submission Type, Submission Review Type, Billable flag, and Type Qualifier, and then click the submit button. |
|
Notify IRB |
Select a Submission Type, Submission Review Type (required), and Committee; browse for and select a file for the File Name field, and type to enter a comment in the Comment field as desired; then click the submit button. This is used by the Researcher to inform the IRB of an event or new information about the protocol that does not require an amendment. |
|
Request for Data Analysis Only |
The Investigator submits a request to notify the Committee that the human participant portion of the study is complete and only data analysis remains to be completed. A comment is included, and a Protocol Submission record is created. |
|
Request for Suspension |
Investigator submits a request to suspend the study. A reason for the suspension is required. Creates protocol submission record. |
|
Request for Termination |
Type a reason for the termination request, then click the submit button. |
|
Request to Close |
Investigator submits a request to the IRB to close with a comment. Protocol will be submitted to the committee which approved the protocol. |
|
Request to Close Enrollment |
Investigator submits a request to close enrollment of participants into the study. A comment is included. Creates protocol submission record. The request to close enrollment requires action by IRB Administrator to close enrollment. |
|
Response Approval |
IRB Administrator records approval action resulting from a review of the Researcher’s response to a previously-reviewed protocol that required revisions. Enters the approval date, risk level and comments. Expiration date will be generated based on approval date, but can be modified. Present approval letter for review and to mark final. |
|
Submit for Review |
Submit a new protocol/ Amendment/ Renewal/ Response to IRB office for review. Must select Submission type (New/Amendment/Renewal/Response) and Review type (Full/ Exempt/ Expedited). Entering committee, schedule and reviewers is optional. Checklist is required for exempt or expedited. Submission date set to current date. |
|
Suspend |
IRB Administrator marks protocol as suspended. Enter comments, reviewer comments and action date. |
|
Suspend By DSMB |
Protocol is suspended by Data Safety Monitoring Board (DSMB). IRB Administrator enters comments, reviewer comments and action date. |
|
Terminate |
Enter comments that summarize the reason for the termination, then select an action date for the termination to become effective. |
|
Undo Last Action |
Type to enter comments that summarize the purpose of undo-ing the last action, then click the submit button. |
|
Withdraw Protocol |
Type a textual reason for the withdrawal, then click the submit button to withdraw a Protocol application from review. The PI and any Correspondents are automatically notified. |
Reviewer View
When the Protocol document is accessed by an IRB Administrator or Committee Member (Reviewer), they are able to view the protocol application (including associated attachments, comments and history) that has been assigned to them and placed on the committee’s agenda.
Role Configuration: The roles and equivalent job titles may vary by implementing institution. In general, however, Reviewers include compliance committee members that have been assigned to review protocol applications, and IRB administrative staff are typically involved in coordinating committee review. KC is flexible in terms of the configuration of roles and associated permissions, and therefore can be tailored to your institution’s unique business rules.
Action Requests: Reviewers are able to select from a list of possible action options which appear as subsections in the Request an Action tabbed section of the Protocol Actions page. The Committee document, and its integrated Meeting documents, are also used to record information pertaining to review activities, such as vote tracking, determinations and decision information. The ability to enter comments is possible for nearly every available action.
Action List Notifications: Notifications are generated automatically by the system to alert Reviewers when a new Protocol document has been submitted and assigned for online review – either to an individual Reviewer, or by way of the Committee meeting agenda. The user’s action list will always display expected actions requested, and from it, the Protocol documents may be directly accessed.


 key from a previous
field) to relocate the cursor to the field, and then type (or paste from
virtual clipboard) to enter text in the box as necessary to provide the
appropriate information.
key from a previous
field) to relocate the cursor to the field, and then type (or paste from
virtual clipboard) to enter text in the box as necessary to provide the
appropriate information. button to
confirm.
button to
confirm.

 Common Protocol Document Life Cycle
Stages By User Type/Status
Common Protocol Document Life Cycle
Stages By User Type/Status