Modify Submission Request
This action allows the IRB Administrators to change the protocol submission review type. The review type may be set by the investigator during initial protocol submission. This action becomes available only after the protocol has been submitted to the IRB. The system will present pre-defined checklists for Expedited and Exempt reviews if the review types changed to Expedited or Exempt.
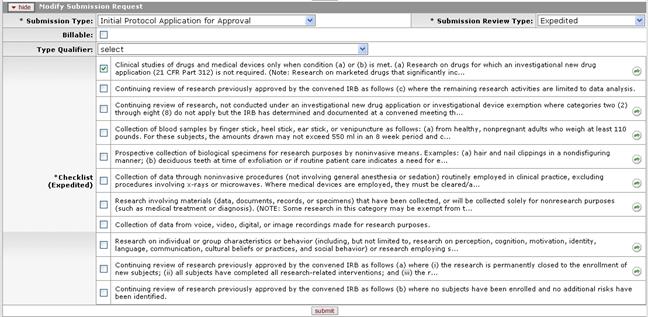
Figure 1173 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Modify Submission Request (Submitted Protocol, Expedited – Status: Submitted to IRB)
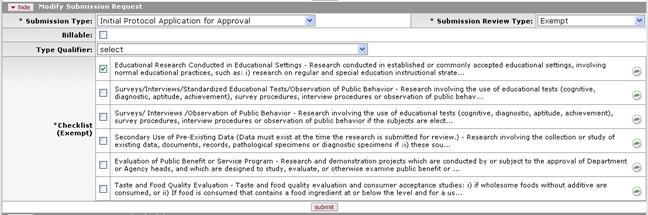
Figure 1174 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Modify Submission Request Layout (Exempt Checklist Example)
Table 382 Protocol Document, Protocol Actions Page, Request an Action Section, Available Actions – Modify Submission Request Field Descriptions
|
Field |
Description |
|
Submission Type |
Select one of the following options from the list: • Initial Protocol Application for Approval • Continuing Review/Continuation without Amendment • Amendment • Response to Previous IRB Notification • Continuing Review/Continuation with Amendment Use the drop-down |
|
Submission Review Type |
Select one of the following options from the
list: Full, Expedited,
Exempt, Limited/Single Use, IRB Review not required, Response, or
FYI. Use the drop-down |
|
Billable |
To indicate that the sponsor is billed for protocol
review, click within the checkbox
|
|
Type Qualifier |
Select one of the following from the list:
Request for Eligibility Exception, Training Certification, Unanticipated
Problems, DSMB Report, Annual Report, Annual Scheduled by IRB,
Contingent/Conditional Approval/Deferred Approval/Non-Approval,
Eligibility Exceptions/Protocol Deviations, AE/UADE, Compliant, Deviation,
Protocol-related COI Report, or Self report for Noncompliance. Use
the drop-down |
|
Checklist (Type) |
Differs depending on the action (for example, Exempt or
Expedited). Click the green arrow |
|
Submit |
Click the submit |




 symbol to view full text in a
separate browser window. Click within the
symbol to view full text in a
separate browser window. Click within the  button to generate the action
request.
button to generate the action
request.