Required Fields for Saving Document
A self-explanatory section, the four fields collect a minimum amount of basic information to uniquely identify the new document. Once filled out, you have the ability to click the global save button at the bottom, center of the screen. A successful save provides you with the ability to sign out of KC and return at a later time to continue to populate other fields in the Protocol document.
|
|
For more information about initiating a document, see “Initiating a Document” on page 107 in Common E-Doc Operations. |
|
|
For more information about saving a document, see “Saving a Document” on page Error! Bookmark not defined. in Common E-Doc Operations. |
|
|
For more information about searching for a saved document, see “Searching for a Document” on page 114 in Common E-Doc Operations. |
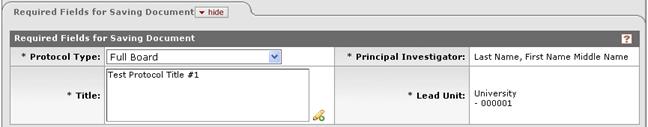
Figure 1103 Protocol Document Protocol Page - Required Fields for Saving Document Section
Table 319 Protocol Document Protocol Page - Required Fields for Saving Document Section Descriptions
|
Field |
Description |
|
Protocol Type |
Required. Indicates the intended use of the
protocol. This may imply the level of review the protocol likely
will require, however, the final determination is typically made by the
IRB prior to review. Select one of the following options:
Emergency Use, Exempt, Expedited,
Full Board, Humanitarian Use Device (HUD), Single Patient Use or
Standard. By default, the system defaults to Standard. Use the
drop-down
|
|
Title |
Required. Click within the text box (or
press the tab |
|
Principal Investigator |
Required. Click the lookup |
|
Lead Unit |
Required. By default, this is the primary home
unit of the PI, as defined in the KC Person
table. It is automatically populated after selection of the
Principal Investigator in the preceding field. Type the suspected
value in the box and click the direct
inquiry
|
|
|
The Description field in the Document Overview section is the one field outside this section that is also required to save a draft of a new Protocol document. |

 menu by clicking the down arrow to display the list, and then click on
an item in the list to highlight and select it to populate the box with
your selection.
menu by clicking the down arrow to display the list, and then click on
an item in the list to highlight and select it to populate the box with
your selection.
 key
from a previous field) to relocate the cursor to the field, and then type
(or paste from virtual clipboard) to enter text in the box as necessary to
provide the appropriate information. Click the add note
key
from a previous field) to relocate the cursor to the field, and then type
(or paste from virtual clipboard) to enter text in the box as necessary to
provide the appropriate information. Click the add note  icon to
view/edit/paste text in a new browser window, then click the continue
button to return to the text entry field in the document. After
saved, click the green arrow
icon to
view/edit/paste text in a new browser window, then click the continue
button to return to the text entry field in the document. After
saved, click the green arrow  symbol to view full text in a
separate browser window.
symbol to view full text in a
separate browser window.
 icon or
click the
icon or
click the 