Special Review
The Special Review page has a single tabbed section also labeled Special Review that provides the standard add line item functionality described in the Common E-Doc Operations section of KC Overview.
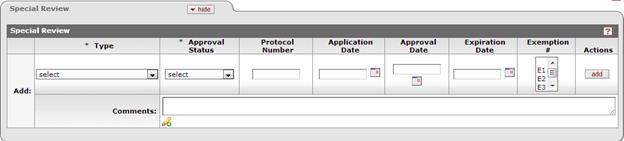
Figure 196 Proposal Development Document > Special Review Page > Special Review Section – Example
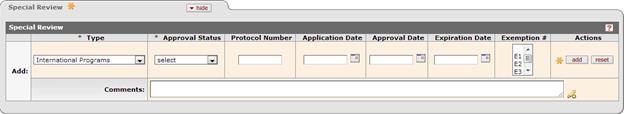
Figure 197 Proposal Development Document > Special Review Page > unlinked module type example
Table 77 Proposal Development Document > Special Review Page > Special Review Section – Field Descriptions
|
Field |
Description |
|
Add |
The line where you make your entries and selections and click the add button, which adds them as numbered line items below. |
|
Type |
Once a selection is made, additional fields are
required. The type indicates which special review, if any, is
relevant to the document. Type selections include use of human
participants, animal usage, biohazard materials, foundation relations,
international relations, etc. Click the down arrow |
|
Approval Status |
|
|
Protocol Number |
The unique identifier assigned to the protocol submitted for regulatory review. The protocol # is the number assigned to the protocol submitted by the PI to the institution's relevant committee (e.g. IACUC, IRB, etc.). A unique number assigned by the institution and/or compliance committee to a specific research Protocol. Depending on implementation, you may type the value if
known in the box, or you may be required to search for and select it and
populate the box automatically by using either the lookup |
|
Application Date |
Enter the date the protocol was submitted to the
regulatory committee. The application date is the date the PI submitted a
protocol/request for permission to the relevant institutional
committee/person for special Review. Enter the date in mm/dd/yyyy format or select it
using the date selector by clicking on the calendar icon |
|
Approval Date |
Enter the date the protocol was approved by the
regulatory committee. The approval date is the date the protocol/request
for permission was approved by the relevant institutional
committee/person. Enter the date in mm/dd/yyyy format or select it
using the date selector by clicking on the calendar icon |
|
Expiration Date |
Enter the date, after which, the existing protocol
expires. Enter the date in mm/dd/yyyy format or select it from the
calendar selector to specify the date this special review will
expire. Enter the date in mm/dd/yyyy format or select it
using the date selector by clicking on the calendar icon |
|
Exemption # |
Select one of six categories of exempt research specified by the Common Rule (45 CFR 46.101(b). If the proposed project is exempt, as defined by the different institutional committees, an exemption number is generally issued, and is entered into this field. Scroll the spin box to view the list items from which to select. |
|
Comments |
Type to enter internal notes relevant to the protocol or special review item. |
|
Actions |
Add is presented in the initial header line. After line items have been added, the delete button appears in this column to allow the line to be removed by clicking the delete button. |
Special Review linking Enabled to Protocol Documents
The Special Review page is integrated with the Human Participants (IRB) and Animal Care (IACUC) modules to allow the linking of Proposal Development, Institutional Proposals, and Award documents to IRB Protocol and IACUC Protocol documents.
When the system has been implemented to enable the protocol linkages, users will need to do one of the following to complete the required fields:
1. Use the lookup to locate and select an existing protocol record that conforms to the sponsor requirements (appropriate status and valid dates)
2. Start a new protocol record to support the proposal submission.
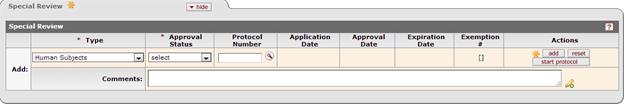
Figure 198 Proposal Development Document > Special Review Page > IRB Module Link Enabled– Example. The user must search the IRB Protocol module for a record; the date fields and the exemption numbers are not presented to maintain. If a record does not exist, the user may begin a new protocol record by selecting the “start protocol” button.
|
|
Option 1:Link to an Existing Protocol document:
|
|
1. |
In the Type field,
select ‘Human
Subjects’ or ‘Animal
Usage’ from the list. Use the
drop-down The Protocol Number
column field becomes editable with a lookup icon |
|
2. |
Enter the document
number for the Protocol document you want to link to, or click the
lookup The Approval Status, Application Date, Approval Date, Expiration Date and Exemption # fields are automatically populated based on your selection when the data exists. |
|
3. |
Enter comments as
desired. Click within the text box (or press the tab |
|
4. |
Click the add
Your selection/entry is added as a numbered line item row below the Add row. Viewing The Linked Document |
|
5. |
Click the view
The Protocol document appears in a new browser window. |
|
|
Option 2: Start a New Protocol document:
|
|
1. |
In the Type field,
select ‘Human
Subjects’ or ‘Animal
Usage’ from the list. Use the
drop-down The Protocol Number
column field becomes editable with a lookup icon |
|
2. |
Click the ‘start protocol’ button
|
|
3. |
Enter comments as
desired. Click within the text box (or press the tab |
|
4. |
Click the add
Your selection/entry is added as a numbered line item row below the Add row. Viewing The Linked Document |
|
5. |
Click the view
The Protocol document appears in a new browser window. |
 to display the list and
click on an item in the list to highlight and select it to populate the
box with your selection.
to display the list and
click on an item in the list to highlight and select it to populate the
box with your selection.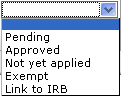 Required if a type is selected.
Select from the list to indicate if the special review relevant to the
document is pending approval, or already approved.
Required if a type is selected.
Select from the list to indicate if the special review relevant to the
document is pending approval, or already approved. or
or  functions.
functions. .
.
 menu by clicking the down
arrow to display the list, and then click on an item in the list to
highlight and select it to populate the box with your
selection.
menu by clicking the down
arrow to display the list, and then click on an item in the list to
highlight and select it to populate the box with your
selection. key from a previous field) to
reposition the cursor so that it is within the field, and then type (or
paste from virtual clipboard) to enter text in the box as necessary to
provide the appropriate information. Click the expand text
key from a previous field) to
reposition the cursor so that it is within the field, and then type (or
paste from virtual clipboard) to enter text in the box as necessary to
provide the appropriate information. Click the expand text
 icon to display a
pop-up window with an expanded text entry area if you want more screen
real estate to type in, and then click the continue button to close
the window and return. After text has been entered and saved, click
the green arrow
icon to display a
pop-up window with an expanded text entry area if you want more screen
real estate to type in, and then click the continue button to close
the window and return. After text has been entered and saved, click
the green arrow icon to read it in its entirety in the larger pop-up window, and then
click the close button to close the window and
return.
icon to read it in its entirety in the larger pop-up window, and then
click the close button to close the window and
return. button in the
Actions column.
button in the
Actions column. button in the
Actions column for the numbered line item row of the document you want to
view.
button in the
Actions column for the numbered line item row of the document you want to
view.